“Brooks staffing is amazing. Definitely take care of their clients and everything is well maintained. I would recommend this place to anyone trying to find a new way of life and work through any kind of trauma.”
-Dominic, via Google Reviews
“This place saved my life. Not only is the property absolutely beautiful, but the dedicated staff are some of the greatest humans I’ve ever met. They nurture an environment of support, healing and recovery.”
-Carly, via Google Reviews

Founded with Love. Rooted in Nature.
LEARN TO NAVIGATE LIFE’S WATERS WITH CLARITY OF MIND, BODY, AND SPIRIT
Healing from substance abuse can be a lonely journey, but you don’t have to do it all on your own. At our drug and alcohol treatment facility in Tennessee, Brooks Healing Center, you matter. We’re here to lend you love, support, and acceptance on your journey to find yourself again.
“My experience at Brooks was forever life changing. The staff is amazing, the food is amazing, the beds are comfortable and everyone is so very compassionate, empathetic, authentic and honest and helped me love myself again to a road of my personal life recovery. If you know someone that’s ready to start their recovery journey and tired of being tired then this place has all the tools available to start their foundation.”
-Jeffery S., via Google Reviews
Your Foundation for Lasting Recovery
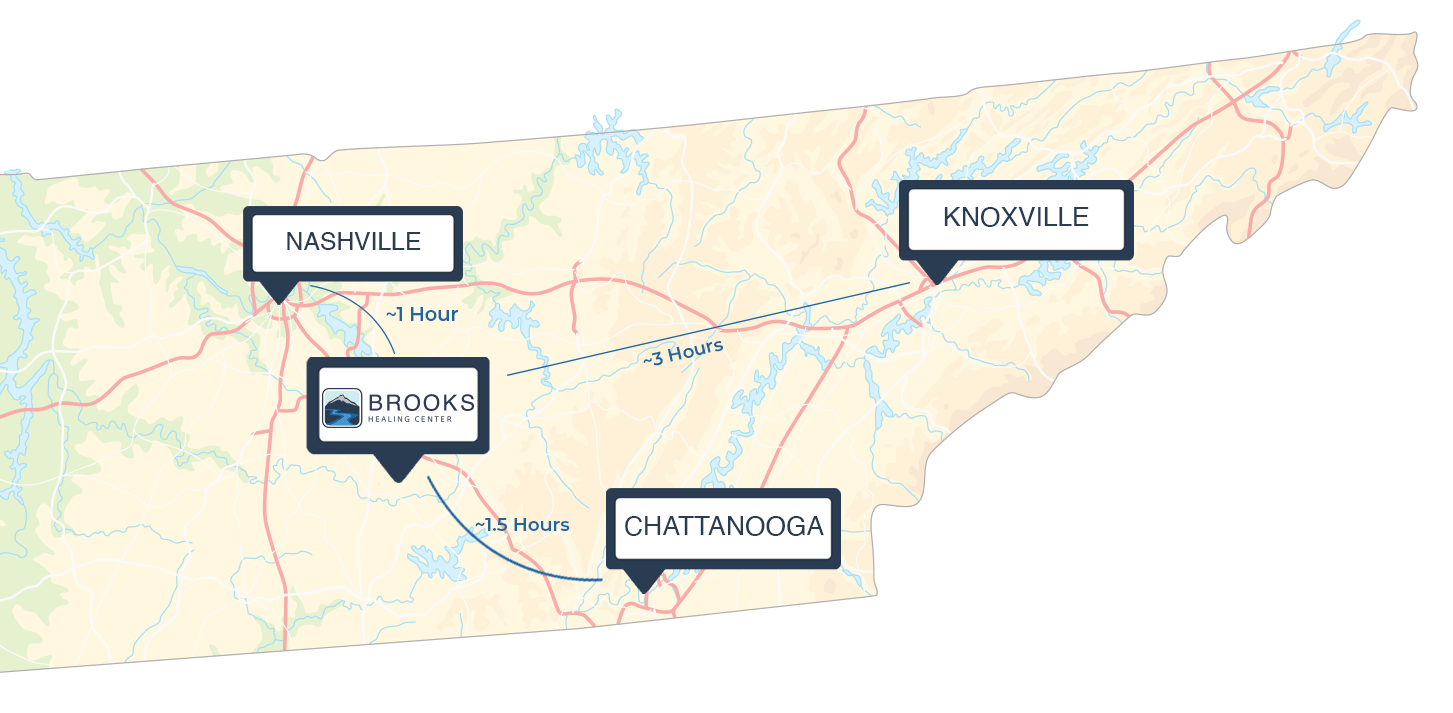
- Close enough to city comforts, yet separated enough from the real world so you can heal in peace
- You will find yourself in a relaxing and supportive environment that helps you leave the pressures of life behind as you take your first steps on the road to recovery

This is Tyler.
During his own recovery journey, he’s seen how insincere some people can be.
Now, he’s made it his life mission to share the good news…
There are still good people out there who will keep their promises and provide the best care available. They’re at Brooks Healing Center, located in Normandy, TN.
Together, we’ll help create your own path to full recovery from substance use and the internal struggle that you’ve been fighting for a long time.
Verify Your Insurance Benefits
Send Us a Message